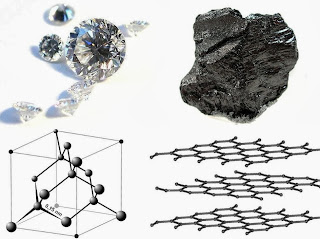
Next we discussed an interesting element: carbon. Carbon can come in may different forms, each with vastly different properties.
Handout: Graphite to Diamonds
Bohr-Rutherford Diagrams
Learning Goals: Understand the structure of atoms.Success Criteria: You can draw the Bohr-Rutherford diagrams for the first 20 atoms on the periodic table.
First you must know how to find the number of protons, neutrons and electrons in each atom. Here's a worksheet for you to practice:
Handout: Subatomic Particles Worksheet
Next we went over how to draw Bohr-Rutherford diagrams. Here are the steps:
- Determine the number of protons and neutrons.
- Write it in the middle (nucleus.)
- Determine the number of electrons.
- Put the electrons in concentric shells (orbitals)
- First orbital can hold up to 2 electrons.
- Second orbital can hold up to 8 electrons.
- Third orbital can hold up to 8 electrons.
- Fourth orbital can hold up to 8 electrons.
Handout: Bohr-Rutherford Diagrams
Here's an example: http://www.youtube.com/watch?v=wy83UlGQpWw
Here are the solutions:
Homework: Complete the handouts and answer the questions to find patterns with these diagrams.
 |
| Dr. Manhattan from Watchmen has a Bohr-Rutherford diagram of hydrogen on his forehead! |

No comments:
Post a Comment