Success Criteria: You can draw the Bohr-Rutherford diagram for ions and show how they bond.
Today we reviewed som Bohr-Rutherford diagrams and I showed you what Lewis dot diagrams are. These diagrams are only concerned with the outer shell of each atom. Here are some examples:
We spent some time discussing the patterns that we see. All group 1 atoms have one electron in the outer shell. All nobel gases have complete outer shells.
Here are the handouts for today:
Here's the main idea you should get out of these handouts: ionic bonds form when a metal loses one or more electrons and a non-metal gains the lost electrons.
 |
| The Bohr-Rutherford diagrams. |
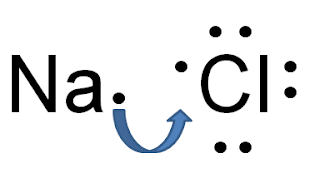 |
| The Lewis dot structures. |
 |
| Positive and negative ions attract each other and join together forming ionic compounds. |
Homework: Complete the handout on Bohr-Rutherford diagrams for ions.
By popular demand, here are the lyrics to the periodic table song!
Video: Periodic Table Song
Written, Directed, Produced, Edited and Sung by Mitchell Moffit.
Based on the "Can-Can" music, by Offenbach.
LYRICS:
There's Hydrogen and Helium
Then Lithium, Beryllium
Boron, Carbon everywhere
Nitrogen all through the air
With Oxygen so you can breathe
And Fluorine for your pretty teeth
Neon to light up the signs
Sodium for salty times
Magnesium, Aluminium, Silicon
Phosphorus, then Sulfur, Chlorine and Argon
Potassium, and Calcium so you'll grow strong
Scandium, Titanium, Vanadium and Chromium and Manganese
CHORUS
This is the Periodic Table
Noble gas is stable
Halogens and Alkali react aggressively
Each period will see new outer shells
While electrons are added moving to the right
Iron is the 26th
Then Cobalt, Nickel coins you get
Copper, Zinc and Gallium
Germanium and Arsenic
Selenium and Bromine film
While Krypton helps light up your room
Rubidium and Strontium then Yttrium, Zirconium
Niobium, Molybdenum, Technetium
Ruthenium, Rhodium, Palladium
Silver-ware then Cadmium and Indium
Tin-cans, Antimony then Tellurium and Iodine and Xenon and then Caesium and...
Barium is 56 and this is where the table splits
Where Lanthanides have just begun
Lanthanum, Cerium and Praseodymium
Neodymium's next too
Promethium, then 62's
Samarium, Europium, Gadolinium and Terbium
Dysprosium, Holmium, Erbium, Thulium
Ytterbium, Lutetium
Hafnium, Tantalum, Tungsten then we're on to
Rhenium, Osmium and Iridium
Platinum, Gold to make you rich till you grow old
Mercury to tell you when it's really cold
Thallium and Lead then Bismuth for your tummy
Polonium, Astatine would not be yummy
Radon, Francium will last a little time
Radium then Actinides at 89
REPEAT CHORUS
Actinium, Thorium, Protactinium
Uranium, Neptunium, Plutonium
Americium, Curium, Berkelium
Californium, Einsteinium, Fermium
Mendelevium, Nobelium, Lawrencium
Rutherfordium, Dubnium, Seaborgium
Bohrium, Hassium then Meitnerium
Darmstadtium, Roentgenium, Copernicium
Ununtrium, Flerovium
Ununpentium, Livermorium
Ununseptium, Ununoctium
And then we're done!!

No comments:
Post a Comment